Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
Scientists have achieved the first ever energy-positive fusion reaction! So what does that mean?
Our lives probably won't change, but our kids' or grandkids' might.

“Last week at the Lawrence Livermore National Laboratory in California, scientists at the National Ignition Facility achieved fusion ignition. That is creating more energy from fusion reactions than the energy used to start the process. It’s the first time it has been done in a laboratory, anywhere in the world. Simply put, this is one of the most impressive scientific feats of the 21st century. Or, as the president might say…”
That was U.S. energy secretary, Jennifer Granholm, in a press conference this morning, announcing the first ever energy-positive artificially created fusion reaction. Granholm’s implication was met with laughter and applause from the gathered crowd, after which she clarified that U.S. President Joe Biden likely referred to the breakthrough as a "BFD." We’ll leave you to figure out what that might mean. This is a family friendly article, Mr. President.
You’re likely to see countless headlines in the coming days and weeks heralding this as the energy equivalent of the Moon landing, and it might be difficult to parse how much, if at all, this is likely to change our lives. In truth, it is a big deal, but you’re unlikely to feel the effects of it for quite a while. It’s less the energy equivalent of the Moon landing and more like Sputnik. The door to sci-fi energy independence might be opened, but there is a long way to go. Before we get into that, however, a brief physics lesson.
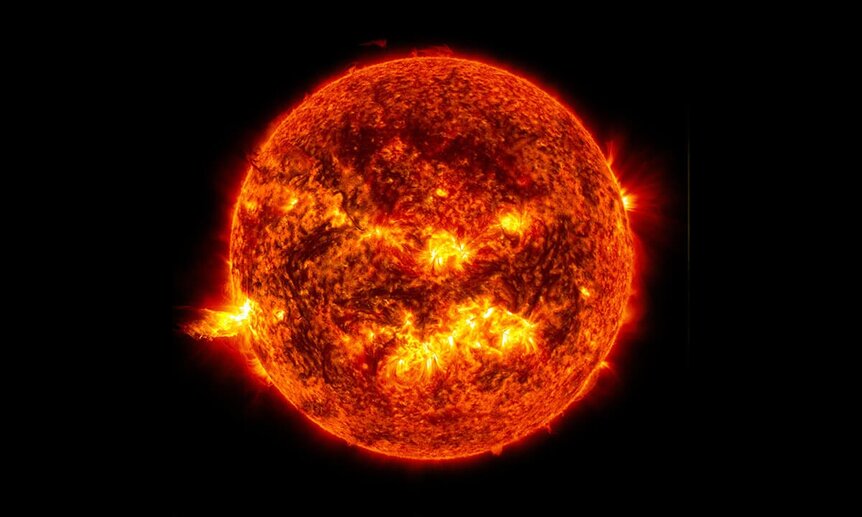
Humans are pretty good at figuring out how to extract energy from things. We’ve developed systems for burning the broken down remains of organic material — that’s where oil and coal come from — and for gathering energy from streams and lakes, sunlight and wind. We’ve even figured out how to capture the energy from decaying radioactive elements and turn it into toaster pastries and podcasts. Despite all of our achievements, the incredible infrastructure we’ve designed and built, the artificial lights, climate control systems, and communications lines all over the planet, all of it pales in comparison to nature’s perfect power plant. Stars.
The Sun, like other stars, is capable of pumping out a nearly immeasurable amount of energy continuously for billions of years. It does this without maintenance or human intervention of any kind. That’s because, despite looking like a massive fire, the Sun isn’t actually burning anything. It’s fusing things. The Sun is the most massive object in the solar system, by a long shot. Jupiter takes second place at roughly 1/10th of 1% the mass of the Sun. It’s not even a contest.
All of that mass means a whole lot of gravity, and all of that pressure pushes the Sun’s materials, mostly hydrogen and helium, toward the star’s center. There, in the core, the pressure is so great that the hydrogen atoms are shoulder to shoulder, with nowhere to go. It’s the cosmic equivalent of making a new friend and becoming so attached to one another that outsiders can long longer identify one without the other. Two individuals become a paired set. It happens with people, and it happens with atoms, under the right conditions.
Under the intense heat — roughly 27 million degrees Fahrenheit — and pressure of a stellar core, the two hydrogen atoms fuse together, becoming one helium atom. The trouble is a single helium atom is not the same mass as two hydrogen atoms. In the fusion, something was lost, or so it seems. But we all know that matter can’t be created or destroyed, so what gives?
Enter Einstein. His most famous equation, and probably the most famous equation ever put to paper, provides the answer. E = mc2 is, of course, only a small part of a much larger equation, but it illustrates what’s happening during fusion quite nicely once you understand the variables. “E” represents the energy of a system, “m” represents the mass of the same system, and “c” is the speed of light.
As we all remember from math class, you can’t change one side of the equation without also changing the other. Einstein’s equation tells us that energy and mass are intimately related, and we can use that relationship to power our blenders. When the two atoms fuse and become one slightly less massive atom, that fusion is accompanied by a corresponding burst of energy, which is equal to the mass lost. When it happens in the Sun, we detect that energy as heat, and it powers photosynthesis and keeps the whole food web going. Whether you’re filling up your car, turning on the lights, or grabbing a midnight snack, it’s sunlight all the way down.
Every energy acquisition technology we have is an attempt to gather that sunlight, the product of fusion 93 million miles away, and turn it into something that can do work for us. A stable fusion reactor would put a small fraction of the power of the Sun in our hands, where we can capture the energy directly. And it would do it without polluting the air, releasing more carbon dioxide, or resulting in excess nuclear waste. It’s clean, it’s powerful, and so far, it has existed only in our fictions or in space.
RELATED: New fusion tech utilizes lasers to bypass sun-like temps and get rid of nuclear waste
Over the last several decades, there have been countless efforts to establish effective fusion reactions, racking up a tab on the order of billions of dollars. The technology has been hailed as the obvious long-term solution to all of our energy needs, but getting there hasn’t been easy. The facility where these recent experiments were carried out got its start in 1997 and has been working toward fusion ever since. In 2014, they were able to produce a small amount of energy, only about what a lightbulb might use during a midnight bathroom trip, by shooting a pellet of hydrogen with high-powered lasers. In August of last year, things at Lawrence Livermore heated up, when scientists produced a reaction which delivered about 70% of the energy that went into it, edging ever closer to a net positive result.
Building on that near-success, scientists cranked up the power of the lasers and refined the fuel pellets. In prior experiments, they found that some energy was lost due to uneven squeezing pressure from the lasers, resulting in some of the hydrogen fuel sneaking out the sides without being fused. Their adjustments intended to correct that problem. Then, at 1:03 a.m. on Dec. 5 of this year, scientists at the National Ignition Center fired 192 lasers at a small pellet of frozen hydrogen encased in a diamond cylinder.
The lasers entered at the top and the bottom, causing a rush of X-rays that compressed the size of the hydrogen pellet. In a fraction of a second, 2.05 megajoules of energy were fired at the pellet and 3 megajoules came out the other side of that reaction. For the first time in human history, we have achieved an energy-positive fusion reaction.
There’s no denying this is, in fact, a BFD. But we shouldn’t start shutting down conventional energy infrastructure just yet. Assuming the experiment can be repeated, it needs to scale significantly before you’ll have that hot new fusion electricity coursing through your home. Three megajoules might sound like a lot of power, but it really isn’t. The average consumer in the United States uses about 36 gigajoules — that’s 36 thousand megajoules — in a year. At that rate, a single household could gobble up 3 megajoules in about a quarter of an hour without too much effort.
Additionally, there’s a problem with the fuel pellets. So far, scientists have relied on deuterium and tritium, two rare and heavy forms of hydrogen for their reactions. Unfortunately, tritium isn’t exactly easy to get your hands on. In fact, calling it rare would oversell how much of it there is. Natural tritium forms as a result of cosmic radiation interacting with gases in the atmosphere, and it’s estimated there are only about 7.3 kilograms of natural tritium in the world at any given time. We can and do manufacture it, but it’s measured in grams per year and requires a nuclear reactor to produce. It’s not the sort of fuel source to build a global society on top of unless we can figure out a way to produce it way more efficiently, and without using so much energy that it eats away the surplus from the fusion reactions.
Probably, this discovery won’t lead to any significant changes in the short-term, but it could lay the foundation for continued innovation and scientific discovery, which might one day allow us to hold the power of the Sun in our figurative hands.